The dreaded “Red List” and “Black List” (even the colors and short-form names spark fear!) commonly refer to FDA’s enforcement tool, “Import Alert”, an FDA-directive that is intended to inform the FDA’s field staff and the public that the FDA has enough evidence or other information to allow for “automatic detention” or “Detention Without Physical Examination” (DWPE) of products that appear to violate FDA’s laws and regulations.
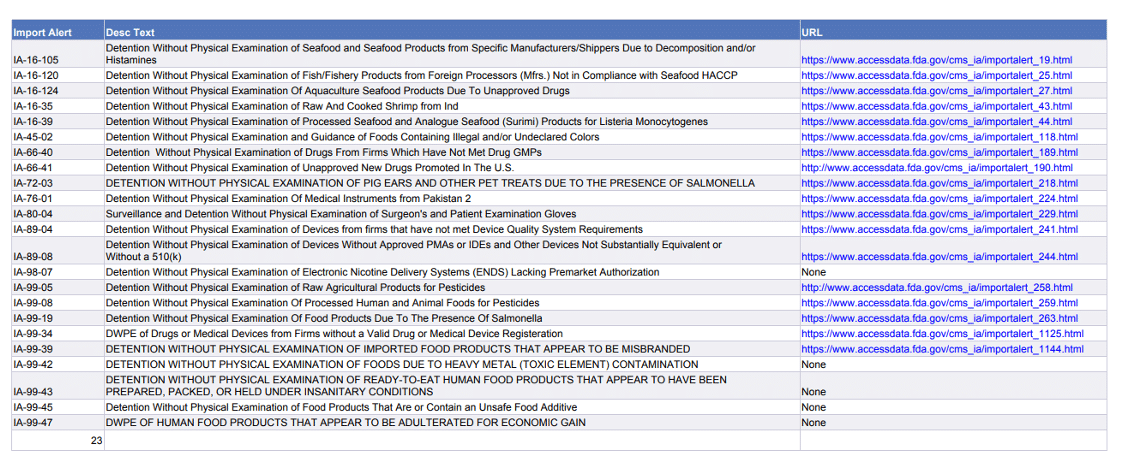
The Agency updates Import Alerts routinely. This week, for instance, FDA Import Alerts on the following products have either been newly issued or modified:
It is important to note that violations could be product-related, or based on violations associated with the manufacturer, shipper, and/or other information, and can span across all FDA-regulated categories: foods, dietary supplements, cosmetics and personal care products, medical devices, Over-the-Counter (OTC) drugs, animal and veterinary products, etc.
In layman’s terms, an Import Alert placement means that a Company has received a series of violations (and opportunities to correct these violations) e.g. via Import Detentions, Import Refusals, FDA Inspections, 483s, Warning Letters, etc., and that FDA has determined that there exists a presumption of violation for the facility and/or products such that all imported goods are automatically detained. In other words, FDA “passes the baton” to the importer to demonstrate compliance.
FDA also plays a game of “Red Light Green Light” when it comes to Import Alert placements. For instance, firms and/or products on the “Red List” (also known as the “Black List”) of an Import Alert are subject to DWPE. This is in contrast to firms and/or products that are on the “Green List” who have met the criteria for exclusion. There are also some Import Alerts that include a “Yellow List” of firms, products and/or countries that are subject to increased surveillance due to the nature of the violations warning additional field examination or analysis. Placement on Import Alert results in significant delays at the time of import, including a routine DWPE process where an importer receives an automatic FDA Notice of Detention, and must provide information demonstrating shipment compliance. This also results in additional and steep testing fees, entry processing delays, and significant variability in getting products to distributors/customers.
While some products may continue to be imported into the U.S. notwithstanding an Import Alert placement if the importer can demonstrate the shipment is in compliance, the only way to eliminate the Import Alert (and all the nuisances that come with it!) is via a Petition for Removal process. This process permanently removes a facility and/or product from the Import Alert. It is important to first identify the Import Alert removal strategy based on the conditions of placement, and FDA’s requirements for removal which routinely involve investigation, documentation, consistent and effective implementation of corrective actions, and consecutive clean entries (number of clean entries varies pending the nature of the Import Alert). Thereafter, drafting and providing FDA appropriate and complete documentation along with a robust Petition outlining the laws, regulations and facts, are critical to a successful Removal and to clearing the path for smoother marketplace entry.
For more information on seeking removal or exemption of your Company from an FDA Import Alert, please contact us at info@garg-law.com.